Abstract
Purpose
The aim of this study was to describe the changes in respiratory system compliance and other measures of respiratory mechanics associated with peritoneal insufflation (12 mmHg pressure) with carbon dioxide (PNP12) and 20° Trendelenburg positioning (TDG20) in pediatric patients undergoing laparoscopic surgery for abdominal cryptorchidism.
Methods
Twelve subjects with abdominal cryptorchidism undergoing orchiopexy were enrolled in the study. General anesthesia was conducted with sevoflurane/O2/air, fentanyl, and rocuronium. Pressure-controlled ventilation with a peak inspiratory pressure (PIP) of 10-15 cm H2O and a positive end-expiratory pressure of 5 cm H2O was titrated to achieve a tidal volume (VT/kg) of 6-10 mL·kg−1 and end-tidal carbon dioxide (EtCO2) of 35-40 mmHg. Adjustments of PIP and respiratory rate (RR) were made to maintain the initial VT/kg and EtCO2 < 50 mmHg. Measurements of weight-corrected dynamic compliance (Cdyn/kg), VT/kg, and EtCO2 were recorded at baseline, after PNP12, at TDG20, and again after deflation and return to the level position.
Results
Adjustments in PIP were required in all subjects to maintain the target VT/kg. The Cdyn/kg decreased 42% (95% confidence interval [CI]: 30 to 51; P < 0.001) after PNP12, and it remained below baseline until deflation. The TDG20 caused only minimal additional reductions in Cdyn/kg (10% decrease; 95% CI: 0 to 19; P = 0.048). The VT/kg decreased 42% (95% CI: 31 to 52; P = 0.048) with PNP12, and after TDG20, it decreased a further 10% (95% CI: 4 to 19; P = 0.038). After deflation, the VT/kg increased by 56% (95% CI: 28 to 90; P = 0.001) and was then adjusted back to the initial values.
Conclusion
The PNP12 significantly decreases Cdyn/kg and VT/kg in pediatric patients. The use of TDG20 produces a relatively minor additional impact in respiratory mechanics. This study emphasizes the need to adjust ventilator settings to maintain normal gas exchange during this procedure.
Résumé
Objectif
L’objectif de cette étude était de décrire les changements de compliance du système respiratoire et d’autres mesures de la mécanique respiratoire associés à une insufflation péritonéale (pression de 12 mmHg) à l’aide de dioxyde de carbone (PNP12) et d’une position de Trendelenburg à 20° (TDG20) chez les patients pédiatriques subissant une chirurgie par laparoscopie pour cryptorchidie abdominale.
Méthode
Douze patients atteints de cryptorchidie abdominale subissant une orchidopexie ont pris part à l’étude. L’anesthésie générale a été réalisée à l’aide de sévoflurane/O2/air, de fentanyl et de rocuronium. Une ventilation en pression contrôlée avec une pression inspiratoire maximale (PIP) de 10-15 cm H2O et une pression positive télé-expiratoire de 5 cm H2O a été titrée afin d’obtenir un volume courant (VT/kg) de 6-10 mL·kg−1 et un dioxyde de carbone télé-expiratoire (EtCO2) de 35-40 mmHg. La PIP et la fréquence respiratoire (FR) ont été ajustées afin de maintenir le VT/kg initial et un EtCO2 < 50 mmHg. Les mesures de la compliance dynamique corrigées selon le poids (Cdyn/kg), du VT/kg et du EtCO2 ont été enregistrées avant la chirurgie, après la PNP12, lors de la TDG20, puis après dégonflement et retour à la position à plat.
Résultats
Il a fallu ajuster la PIP chez tous les patients afin de maintenir le VT/kg cible. La Cdyn/kg a diminué de 42 % (intervalle de confiance [IC] 95 %: 30 à 51; P < 0,001) après la PNP12, et elle est demeurée inférieure aux valeurs de base jusqu’au dégonflement. Les réductions additionnelles de la Cdyn/kg causées par la TDG20 étaient minimales (réduction de 10 %; IC 95 %: 0 à 19; P = 0,048). Le VT/kg a baissé de 42 % (IC 95 %: 31 à 52; P = 0,048) avec la PNP12, et après la TDG20, il a baissé de 10 % supplémentaires (IC 95 %: 4 à 19; P = 0,038). Après le dégonflement, le VT/kg a augmenté de 56 % (IC 95 %: 28 à 90; P = 0,001) puis a ensuite été réajusté aux valeurs initiales.
Conclusion
Une PNP12 réduit significativement la Cdyn/kg et le VT/kg chez les patients pédiatriques. L’utilisation d’une TDG20 a un impact supplémentaire relativement mineur sur la mécanique respiratoire. Cette étude souligne le besoin d’ajuster les paramètres du ventilateur afin de maintenir un échange gazeux normal pendant cette intervention.
Similar content being viewed by others
The respiratory effects of Trendelenburg positioning (TDG) during laparoscopic surgery in children are not well studied. Laparoscopic surgery is the primary surgical approach for the diagnosis and treatment of many surgical disorders, including intra-abdominal testes (cryptorchidism).1 When compared with other approaches, the “minimally invasive” nature of laparoscopy is associated with reduced hospital length of stay as well as better cosmetic outcomes.1 Pneumoperitoneum (PNP) induced by carbon dioxide (CO2) insufflation allows for improved surgical visualization and maneuvering of laparoscopic instruments in the abdominal cavity. Trendelenburg positioning optimizes visualization of the pelvic structures and allows for high dissection of the testicular vessels during cryptorchidism surgery.
Marked changes in respiratory mechanics occur after the induction of general anesthesia, with notable reductions in the functional residual capacity (FRC) and respiratory system compliance (Crs), especially with the use of neuromuscular relaxants.2 These changes may be more pronounced in the pediatric population.3 Additional deleterious changes in the pulmonary mechanics during laparoscopic procedures may occur with the use of PNP and TDG, both of which are necessary for the procedure. These changes include: cephalic displacement of the diaphragm, decreased FRC and Crs, and increased airway resistance. If no changes are made to ventilatory parameters, decreased tidal volume (VT) and minute ventilation (MV) may also occur. This, in turn, leads to intrapulmonary shunting with the development of hypoxemia and hypercapnia.4,5 These effects have been well characterized in the adult population,6,7 but there is limited information available in children.8 The available studies in pediatric populations are limited by small sample sizes, heterogeneous procedures involving the upper and lower abdomen, variable positioning, and minimally controlled ventilatory techniques (manual and volume-controlled).6,9
The aim of this study was to detail the changes in respiratory system compliance and other measures of pulmonary function resulting from introducing a 12 mmHg pneumoperitoneum (PNP12) and 20° Trendelenburg positioning (TDG20) under pressure-controlled mechanical ventilation during general anesthesia in pediatric patients undergoing pelvic laparoscopic surgery for cryptorchidism.
We hypothesized that induced PNP12 would produce significant and clinically relevant changes in respiratory system dynamic compliance in anesthetized pediatric patients using pressure-controlled ventilation, and that a further decrease in dynamic compliance would occur after the addition of TDG20.
Methods
After approval from the Children’s Hospital of Eastern Ontario Research Ethics Board (November, 2009), informed consent was obtained from the parents of children undergoing laparoscopic surgery for cryptorchidism from Dec 1, 2009 to Jan 30, 2012. Demographic data collected included: age, weight, history of prematurity, gestational age at birth, mechanical ventilation at birth (and its duration), the presence of a history of pulmonary disease (bronchopulmonary dysplasia, asthma, etc.), congenital heart disease, and syndromes or other pathologies. Our intent was to avoid enrolling patients with severe and/or active cardiopulmonary disease. Any perioperative adverse events were recorded, such as subcutaneous emphysema, hypotension, bradycardia, pneumothorax, gas embolism, desaturation (pulse oximetry [SpO2] < 92%) or hypercapnia (end-tidal CO2 > 55 mmHg).
Surgical protocol
The surgical team performed a laparoscopy using a Hasson technique for the umbilical corkscrew port insertion (a three-port approach), 5-mm instruments and a 30° lens. Insufflation pressures were set at 12 mmHg and continuously measured using the GS 1002 402 Insufflator (ConMed Linvatec, Largo, FL, USA).10 The patient was placed in a TDG20 along with slight rotation of the table to the appropriate side to expose the left or right internal inguinal rings. The technique chosen for orchiopexy was performed according to surgical findings, with some patients undergoing a laparoscopic-aided orchiopexy and others undergoing a Fowler-Stephens staged orchiopexy.11
Anesthesia protocol
Thirty minutes before the procedure, the patients were premedicated with acetaminophen 20-30 mg·kg−1 po, with or without midazolam 0.3-0.5 mg·kg−1 po, according to the anesthesiologists’ preference. Standard noninvasive monitors were applied before the induction of anesthesia.12 Inhalation induction was performed with 60% N2O and 40% O2 and increasing concentrations of sevoflurane until the patients reached stage 3 of anesthesia. The N2O was then discontinued and an intravenous catheter was inserted. Normal saline was used for routine intraoperative fluid management. Anesthesia induction was completed with propofol 2 mg·kg−1 fentanyl 1 µg·kg−1, and rocuronium 0.5 mg·kg−1. Tracheal intubation was performed with a cuffed endotracheal tube (ETT) appropriate for each patient’s size. The ETT cuff pressure was measured with a Portex™ low pressure scale manometer (Portex Ltd, Hythe, Kent, UK) and maintained at 20-25 cm H2O. Maintenance of anesthesia was performed using oxygen and air (F i O2 0.5), sevoflurane, propofol, fentanyl, and rocuronium. Pulmonary ventilation in all patients was supported with a Datex Ohmeda Aestiva®/5 anesthesia machine with a PSVPro SmartVent ventilator (Datex-Ohmeda, Inc. Madison, WI, USA). Baseline ventilatory parameters included pressure-controlled ventilation (PCV), positive end-expiratory pressure (PEEP) of 5 cm H2O, and peak inspiratory pressure (PIP) of 10-15 cm H2O. These parameters were titrated to achieve a VT of 6-10 mL·kg−1 and a respiratory rate (RR) of 12-20 breaths·min−1 and then finally adjusted to achieve an end-tidal CO2 (EtCO2) of 35-40 mmHg and a target SpO2 of ≥ 97 %.
Ventilatory parameters were manually recorded from the ventilator monitor screen. The PSVPro SmartVent ventilator has an inspiratory pressure range of 5-60 cm H2O and a PEEP range of 4-30 cm H2O, with an accuracy of pressure delivery of ± 10% (or ± 3 cm H2O), pressure monitoring of ± 5% (or ± 2 cm H2O), volume delivery of ± 7% (or ± 11 mL), and volume monitoring of ± 9% (or ± 9 mL).
Portex pediatric disposable anesthesia breathing circuits (137 cm in length) with a measured compliance of 1.26 mL·cm H2O−1 and a one-litre bag were used in all cases. No corrections were performed for compliance of the anesthesia circuit. A compact airway module (M-CAiO) in the S/5Anesthesia Monitor (Datex-Ohmeda, Inc. Madison, WI, USA) was used to measure EtCO2 and anesthetic gases. Gas sampling tubing (sidestream technique) was connected at the humidifier filter attached to the ETT. The elbow was eliminated from the breathing circuit after intubation.
After a stabilization period of one to two minutes of mechanical ventilation (stabilization defined as a stable VT for at least four consecutive breaths),the following parameters were measured at each intervention (and without any simultaneous surgical manipulation [see below]): baseline PIP, expired VT, RR, MV, dynamic compliance of the respiratory system (Cdyn), sidestream ETCO2, and SpO2. Dynamic compliance of the respiratory system was calculated with the standard formula: [Cdyn = VT / (PIP − PEEP)], and weight-corrected Cdyn (Cdyn/kg) was calculated by dividing Cdyn by the weight of the subject in kilograms. Peak pressures and respiratory rate were adjusted to meet the targets indicated above. All study variables were measured at the following periods:
-
1.
Baseline: with the patient in the supine position after tracheal intubation and stabilization on the ventilator and before surgical incision.
-
2.
PNP12: after insufflating CO2 to an intra-abdominal pressure of 12 mmHg.
-
3.
Corr 1: Correction 1, after adjusting PIP to return VT to 6-10 mL·kg−1 or within 10% of baseline.
-
4.
After TDG20.
-
5.
Corr 2: Correction 2, during TDG20 but after adjusting PIP to return VT to 6-10 mL·kg−1 or within 10% of baseline.
-
6.
Deflation: after completing the surgical procedure with the patient returned to the supine position with the abdomen deflated.
-
7.
Post-deflation Corr: Post-deflation correction, after returning PIP to baseline.
Subsequent care was at the discretion of the anesthetic team and was not part of the study. Ventilatory adjustments were performed during each period, increasing PIP first by 2 cm H2O at a time until a VT of 6-10 mL·kg−1 or within 10% of baseline VT was obtained, with a maximum PIP of 25 cm H2O and EtCO2 of 35-50 mmHg. Respiratory rate was increased by 2 breaths·min−1 at a time after adjusting VT to maintain an EtCO2 of 35-50 mmHg. Operating room table TDG20 was measured with a Johnson Magnetic Angle Locator (Johnson Level & Tool Mfg. Co., Inc. Mequon, WI, USA). At the end of the procedure, residual neuromuscular blockade was reversed with standard doses of neostigmine and atropine, tracheal extubation was performed, and the patient was transported to the postanesthesia care unit.
The primary endpoint of the study was Cdyn/kg, with secondary endpoints being VT/kg, EtCO2, RR, and SpO2. Demographic data are presented as median and interquartile range [IQR]; variables of interest (PIP, VT/kg, Cdyn/kg, EtCO2, RR and SpO2) are presented as mean (SD) and 95% confidence interval (CI).
The sample size was calculated according to the results of previous studies where a 20% change in Cdyn with anesthetic maneuvers was considered significant.13 In the study of Manner et al.,14 the average change (SD) in compliance from baseline to TDG20 was 9 (9.8) L·cm H2O−1. Assuming no correlation between the compliance at baseline and at TDG20, along with the same degree of variability, a sample of at least 12 subjects would be required to have 80% power to detect a change of this magnitude with the probability of type-I error at 5%.
The primary analysis compared log-transformed Cdyn/kg following various maneuvers using within-subject comparisons in a general linear model with repeated measures. Normality of residuals was assessed using the Shapiro-Wilk test, and homogeneity of variance of the residuals was assessed using Levene’s test. Mauchly’s test was used to evaluate sphericity, and since the assumption of sphericity was violated, the Greenhouse-Geisser (G-G) correction to the test of within-subject effects was applied. Pairwise comparisons of estimated marginal means were used to compare Cdyn/kg by study period. For each period, comparisons were made with each of the other periods, and Bonferroni corrections were used to adjust for these six comparisons. All reported P values are two sided. Similar analyses were performed for the secondary outcome measures. Log transformations were required for VT/kg and EtCO2. To facilitate interpretation, results based on log-transformed quantities that are treated as normally distributed in the models have been transformed back to the original scales. Consequently, absolute differences in the mean on the log-scale, when transformed back to the original scale, must be interpreted as relative changes in the median.13 Confidence intervals must be interpreted similarly. Data were analyzed using SPSS® version 22 (IBM Corp. Released 2013, IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA).
Results
Fifteen subjects consented to participate in the study. Three subjects were excluded from the study after enrolment, two subjects had incomplete data, and one subject did not require laparoscopy because his testicle was palpable at the inguinal canal. Data on demographics and baseline ventilatory parameters are presented in the Table. The median [IQR] age was 6.0 [7.2] years and weight was 21.5 [21.9] kg. All subjects were otherwise healthy.
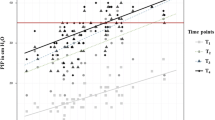
Importantly, to maintain patient stability, the PIP needed to be changed significantly over the course of the procedure (Fig. 1) (P < 0.001). Each PIP, following correction, was significantly different from baseline and from the previous value (P < 0.001 for each change). No leak from the ETT was identified by the anesthesiologists or by the ventilator sensor/alarms.
Peak inspiratory pressure changes (PIP) during laparoscopic surgery. PNP12 = pneumoperitoneum 12 mmHg. Corr 1 = correction 1 is peak inspiratory pressure adjustment following CO2 insufflation to return VT to 6-10 mL·kg−1 or within 10% of baseline; TDG20 = Trendelenburg position 20°; Corr2 = correction 2 is peak inspiratory pressure adjustment following Trendelenburg positioning to return VT to 6-10 mL·kg−1 or within 10% of baseline; Post-def Corr = post-deflation correction. Peak inspiratory pressure adjusted to baseline following deflation and return of the patient to the supine position. Error bars are ± 1 SD. *P < 0.05 compared with baseline
After introduction of PNP12, our primary outcome, Cdyn/kg, decreased by 42% (95% CI: 30 to 51; P < 0.001; Shapiro-Wilk test P > 0.100 in each period), and it remained significantly lower than baseline until deflation (P < 0.001). Lowering of the patient’s head with TDG20 caused a small further reduction in Cdyn/kg (10% decrease; 95% CI: 0 to 19; P = 0.048). After deflation, Cdyn/kg increased and was not significantly different from baseline (P = 0.999). Dynamic compliance of the respiratory system was not significantly associated with age (P = 0.285). To apply the repeated measures model, Cdyn/kg was log-transformed, and since sphericity was violated, the G-G correction was performed with epsilon = 0.37. Log-transformed Cdyn/kg varied significantly with period (P < 0.001) (Fig. 2, untransformed units).
Weight-adjusted dynamic compliance during laparoscopic surgery. PNP12 = pneumoperitoneum 12 mmHg; Corr 1 = correction 1 is peak inspiratory pressure adjustment following CO2 insufflation to return VT to 6-10 mL·kg−1 or within 10% of baseline; TDG20 = Trendelenburg position 20°; Corr2 = correction 2 is peak inspiratory pressure adjustment following Trendelenburg positioning to return VT to 6-10 mL·kg−1 or within 10% of baseline; Postdef Corr = post-deflation correction is peak inspiratory pressure adjusted to baseline following deflation and return of the patient to the supine position. Error bars are ± 1 SD. *P < 0.05 compared with baseline
There were significant changes in VT/kg over the course of the procedure (Fig. 3) (P = 0.0001; as sphericity was violated, the G-G correction was performed with epsilon = 0.2). Baseline VT/kg fell 42% (95% CI: 31 to 52) after introduction of PNP12 (P < 0.001). Peak pressures were adjusted in the ventilator to correct the VT/kg; however, after TDG20, the VT/kg again decreased significantly by 10% (95% CI: 4 to 19; P = 0.038; Shapiro-Wilk test P > 0.050 in each period). It was again adjusted by increasing the PIP to achieve a VT within the acceptable range. At deflation, the VT/kg increased significantly by 56% (95% CI: 28 to 90; P = 0.001), and PIP was readjusted to baseline with a VT/kg that was similar to baseline (P = 0.999).
Weight-adjusted tidal volume during laparoscopic surgery. PNP12 = pneumoperitoneum 12 mmHg; Corr 1 = correction 1 is peak inspiratory pressure adjustment following CO2 insufflation to return VT to 6-10 mL·kg−1 or within 10% of baseline; TDG20 = Trendelenburg position 20°; Corr2 = correction 2 is peak inspiratory pressure adjustment following Trendelenburg positioning to return VT to 6-10 mL·kg−1 or within 10% of baseline; Postdef Corr = post-deflation correction is peak inspiratory pressure adjusted to baseline following deflation and return of the patient to the supine position. Error bars are ± 1 SD. *P < 0.05 compared with baseline
The MV changed significantly during the procedure (P < 0.001; as sphericity was violated, the G-G correction was performed with an epsilon of 0.37) and paralleled the changes in VT/kg. End-tidal CO2 also changed significantly over the course of the procedure (P < 0.001; as sphericity was violated, the G-G correction was performed with an epsilon of 0.35). There was no significant change from baseline after the introduction of PNP12 (P = 0.067; Shapiro-Wilk test P > 0.340 in each period); however, it then increased significantly after placement in the TDG20 (P < 0.001) and remained significantly elevated (P = 0.006), though within the acceptable range (EtCO2 < 50 mmHg) until deflation.
Discussion
Pediatric urological laparoscopic procedures commonly use both PNP and TDG for pelvic procedures, including repair of cryptorchidism studied herein. Therefore, it is clinically important to have a clear understanding of the effects of PNP and TDG. This study showed that Cdyn/kg decreased markedly (42%) after induced PNP12. Nevertheless, the addition of TDG20 produced only a minor effect (10% decrease) in Cdyn/kg and VT/kg, and corresponding changes in VT/kg were also seen. Changes in mechanics were accompanied by a rise in EtCO2. It is important that pediatric anesthesiologists be aware of these changes when caring for children undergoing this type of surgery.
Previous research has reported changes in airway pressure and respiratory system compliance following PNP in infants, children, and adults. Pneumoperitoneum has consistently had a greater effect on respiratory mechanics than TDG.5,6,14 Bannister et al. examined infants less than one year of age who underwent a variety of laparoscopic procedures. Average Cdyn decreased 48%, and VT decreased 33% with PNP 12-15 mmHg.5 In contrast, our study examined a uniform surgical procedure and an older age group. The change in VT documented in their study was lower than our observations, which could be explained by different pulmonary mechanics secondary to age differences in the populations studied, i.e., an infant’s chest wall is more compliant compared with older children.15 Therefore, similar PNP pressures appear to create smaller changes in VT compared with older children. Manner et al. also examined children undergoing a variety of laparoscopic procedures, and their study population was somewhat older than ours.14 They found similar trends, with greater reductions in Cdyn with PNP than with TDG, even though they carried out TDG first. Their group did not correct Cdyn for body size. They used an older generation intensive care unit ventilator (Siemens-Elema Servo 900C) with volume-control mode and a VT target of 10 mL·kg−1 without mention of PEEP, and total intravenous anesthesia technique rather than balanced anesthesia technique. They measured Cdyn with a sidestream spirometer that computes flow and pressure at the proximal end of the ETT and the Y-piece, which likely provides more accurate measurements than would be obtained using our methodology.
In order to keep VT/kg in an acceptable range (6-10 mL·kg−1), PIP was increased by 26% from baseline after PNP, and then increased by 33% from baseline after the patients were placed in the TDG. The effects on PIP are quite similar to those reported by Manner et al. during laparoscopic surgery in pediatric patients using volume-controlled ventilation.14 It is interesting to point out that Manner’s study found a corresponding increase in PIP of 18% after TDG20 and 32% when 12 mmHg PNP was added. In contrast to Manner’s study, our surgical team prefers to perform PNP first and TDG later. We cannot exclude the possibility that TDG followed by induced PNP could have changed the results, for example by altering the respiratory system volume history.
During laparoscopy, changes in compliance result from the increase in elastance of the chest wall proportional to the rise in intra-abdominal pressure caused by the PNP and the associated cephalad shift of the diaphragm during PNP.16 There was a significant decrease in Cdyn after induced PNP of 42 %. Nevertheless, the TDG position had a minimal impact, reducing Cdyn by only 10%. Our finding supports a previous study in adults undergoing laparoscopic gynecologic procedures where TDG20 had no significant change in dynamic lung compliance after ten and 30 min.17 The changes in Cdyn were accompanied by parallel changes in VT/kg. Altering VT would not be expected to affect Cdyn if VT were to remain in the steep portion of the respiratory system pressure/volume curve, but it would affect Cdyn if VT were to approach the upper inflection point. Accordingly, pressure-volume curves were visually interpreted but not formally evaluated during the study.
With the reduction in Cdyn and thus VT, MV falls and gas exchange becomes affected. Studies performed in children and adults have shown that ETCO2 increases following increased insufflation pressures and the associated reduction in compliance.9 In our patients, ETCO2 tended to rise with PNP, and it increased significantly (21%) following TDG20. In spite of this significant increase, ETCO2 always remained within the established “safe” limits (i.e., 35-50 mmHg). The rise in ETCO2, despite augmentation of PIP, suggests that CO2 absorption from the abdomen was occurring. Mattioli et al. found that ETCO2 increased significantly with 10 mmHg PNP in pediatric patients undergoing laparoscopic fundoplication for gastroesophageal reflux.18 Their study included patients with asthma and chronic respiratory symptoms, perhaps accounting for the significant increase in ETCO2 seen at lower insufflation pressures. Nevertheless, the ETCO2 never exceeded 45 mmHg, consistent with our results.
The strengths of this study are the use of relatively more modern anesthesia workstations as well as lung protective modes of mechanical ventilatory support. This strategy uses smaller VTs, typically < 10 mL·kg−1, and PEEP of 5 cm H2O.14,16 In addition, unlike previous studies, we included patients undergoing only a standard single laparoscopic surgical procedure. We used PCV as we considered this to be the most commonly used ventilatory modality in pediatric anesthesia using muscle relaxation. The ventilator was adjusted after each stage according to standard clinical practice; however, we repeated measurements after each adjustment in each stage, and thus, we were able to evaluate the effects of each stage as well as each adjustment. Many advocate for PCV during PNP because it limits airway pressures and may result in a reduced risk of ventilator-induced lung injury.17 It also theoretically improves oxygenation, since it applies greater cumulative pressure to the respiratory system. Nevertheless, several studies found no significant differences in oxygenation and respiratory mechanics when comparing pressure vs volume-controlled ventilation during laparoscopic surgery in adults.18,19 Overall, PCV requires more manipulations than volume-controlled ventilation.
Our study has several limitations. Our study population was healthy but was heterogeneous in terms of age and therefore size. Although we adjusted the Cdyn by weight, Cdyn may be affected by other factors related to age, such as compliance of the chest wall and resistance of the airways. Both the ventilator used and the measurement technique used to obtain Cdyn may also affect our results. We used the Datex Ohmeda Aestiva PSVPro ventilator and monitor to measure the pressures, flows, and volumes. This device measures flow and pressure readings inside the anesthesia machine and not at the patient’s Y-piece. In contrast, Manner et al.14 used a sidestream spirometer that obtains its measurements at the proximal end of the ETT and the Y-piece. Although we did not correct for breathing circuit compliance, we consider our results valid given the acceptable accuracy of the ventilator monitors and taking into account that these are the monitoring parameters available in clinical practice. In addition, we consider the trends in our patients to be of greater importance than the absolute values. The trends should not be appreciably affected by the accuracy of the measuring equipment, with the possible exception that increasing PIP potentially increases the compression volume of the ventilator tubing. We would expect the effect size of this to be minimal.
Another limitation to our study is that we did not measure intrapulmonary pressure, which would be lower than pressure measured at the level of the ventilator due to airway and circuit resistance. As intrapulmonary pressure will be lower than airway pressure, the actual values for Cdyn were likely higher than the measured values. More accurate measurements could be obtained using a calibrated pneumotachograph and an esophageal balloon to approximate pleural pressure more closely. This would provide the opportunity to obtain valuable information regarding the effect of the PNP12 and TDG20 in the lung vs the chest wall. The effects of frequency-dependence on Cdyn were not considered, but respiratory rates were low throughout the study, and significant frequency-dependent effects were unlikely to have occurred. Similarly, at these respiratory rates, it is unlikely that gas trapping occurred. Volume-controlled ventilation with inspiratory pause would facilitate determining static compliance and allow deriving airway resistance. While static compliance would provide more precise information on respiratory mechanics than the Cdyn measurements we obtained, volume-controlled ventilation is not routinely performed in pediatric anesthetic practice, and more specialized respiratory monitoring equipment is not generally available in clinical practice.19 The adjustments performed after each surgical intervention to maintain VT/kg also likely had minor (but unmeasured) effects on our results, but these changes were required for clinical care and allowed us to monitor the ventilator adjustments required to maintain control of SpO2 and EtCO2 during this type of laparoscopic surgery. Our findings illustrate the effects of this procedure on the parameters available to the anesthesiologist during usual clinical practice, and they illustrate the frequency that ventilation must be adjusted by the anesthesiologist during laparoscopic urological surgery. Another potential limitation was the observational nature of our study and the fact that investigators were not blinded to the data being collected. When comparing pressure control vs volume control in homogeneous populations, double-blind randomized controlled trials would be required to identify the optimal ventilator mode for laparoscopic surgery in pediatrics.
New ventilator modes, such as pressure-regulated volume-controlled ventilation could be an interesting alternative to decrease operator manipulation of ventilator parameters. Nevertheless, clinical studies are required to evaluate their use in pediatric populations. Bringing mechanical ventilation modes that are typically found in intensive care unit ventilators to the operating room is promising as they provide set VT at the lowest achievable airway pressure. Pressure-regulated volume-controlled ventilation may potentially minimize operator manipulation and provide a safer method of ventilation for laparoscopic surgery.
In conclusion, this study shows significant changes in Cdyn and lung mechanics during laparoscopic urological procedures in children, and it confirms the need to adjust ventilator settings frequently in order to maintain normal gas exchange during and after surgery. The PNP12 produces the most important changes in respiratory mechanics. The TDG20 did not significantly alter Cdyn, but it did reduce VT significantly and cause a minor rise in ETCO2.
References
El-Anany F, Gad El-Moula M, Abdel Moneim A, et al. Laparoscopy for impalpable testis: classification-based management. Surg Endosc 2007; 21: 449-54.
Lumb AB, Pearl R. Anaesthesia. In: Lumb AB (Ed.). Nunn’s Applied Respiratory Physiology - Sixth Edition. Italy: Elsevier Limited; 2005: 297-326.
Motoyama EK. Respiratory physiology in infants and children. In: Motoyama EK, Davis PJ, (Eds.). Smith’s Anesthesia for Infants and Children - Seventh Edition. Philadelphia: Mosby Elsevier; 2006: 12-69.
Pennant JH. Anesthesia for laparoscopy in the pediatric patient. Anesthesiol Clin North America 2001; 19: 69-88.
Bannister CF, Brosius KK, Wulkan M. The effect of insufflation pressure on pulmonary mechanics in infants during laparoscopic surgical procedures. Paediatr Anaesth 2003; 13: 785-9.
Sharma KC, Brandstetter RD, Brensilver JM, Jung LD. Cardiopulmonary physiology and pathophysiology as a consequence of laparoscopic surgery. Chest 1996; 110: 810-5.
O’Malley C, Cunningham AJ. Physiologic changes during laparoscopy. Anesthesiol Clin North America 2001; 19: 1-19.
Tobias JD, Holcomb GW 3rd, Brock JW 3rd, Deshpande JK, Lowe S, Morgan WM 3rd. Cardiorespiratory changes in children during laparoscopy. J Pediatr Surg. 1995; 30: 33-6.
Bozkurt P, Kaya G, Yeker Y, Tunali Y, Altintas F. The cardiorespiratory effects of laparoscopic procedures in infants. Anaesthesia 1999; 54: 831-4.
Esposito C. Laparoscopic treatment of non-palpable testis. In: Bax K, Georgeson KE, Rothenberg SS, Valla JS, Yeung CK (Eds). Endoscopic Surgery in Infants and Children. NY: Springer-Verlag; 2008: 753-8.
Elyas R, Guerra LA, Pike J, et al. Is staging beneficial for Fowler-Stephens orchiopexy? A systematic review. J Urol 2010; 183: 2012-8.
Merchant R, Chartrand D, Dain S, et al. Guidelines to the practice of anesthesia—revised edition 2014. Can J Anesth 2014; 61: 46-59.
Johnson NL, Kotz S, Balakrishnan N. Continuous Univariate Distributions - Volume I, Second Edition. New York: John Wiley; 1994.
Manner T, Aantaa R, Alanen M. Lung compliance during laparoscopic surgery in paediatric patients. Paediatr Anaesth 1998; 8: 25-9.
Olinsky A, Bryan AC, Bryan MH. A simple method of measuring total respiratory system compliance in newborn infants. S Afr Med J 1976; 50: 128-30.
Fahy BG, Barnas GM, Flowers JL, Nagle SE, Njoku MJ. The effects of increased abdominal pressure on lung and chest wall mechanics during laparoscopic surgery. Anesth Analg 1995; 81: 744-50.
Suh MK, Seong KW, Jung SH, Kim SS. The effect of pneumoperitoneum and Trendelenburg position on respiratory mechanics during pelviscopic surgery. Korean J Anesthesiol 2010; 59: 329-34.
Mattioli G, Montobbio G, Pini Prato A, et al. Anesthesiologic aspects of laparoscopic fundoplication for gastroesophageal reflux in children with chronic respiratory and gastroenterological symptoms. Surg Endosc 2003; 17: 559-66.
Ingimarsson J, Thorsteinsson A, Larsson A, Werner O. Lung and chest wall mechanics in anesthetized children. Influence of body position. Am J Respir Crit Care Med 2000; 162: 412-7.
Conflicts of interest
None declared.
Disclosure of funding
This project was funded by the Department of Anesthesiology of the Children’s Hospital of Eastern Ontario and the CHEO Research Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Victor M. Neira was the principal investigator for the study. Victor M. Neira, Thomas Kovesi, Luis Guerra, Nicholas Barrowman, and William Splinter contributed to the study design. Victor M. Neira and Luis Guerra contributed to data acquisition. Thomas Kovesi, Maria Campos, and Nicholas Barrowman contributed to the data analysis. Victor M. Neira, Thomas Kovesi, Luis Guerra, and Maria Campos contributed to data interpretation. Victor M. Neira was the principal author of the manuscript with contributions from Thomas Kovesi, Maria Campos, Luis Guerra, Nicholas Barrowman, and William Splinter.
Rights and permissions
About this article
Cite this article
Neira, V.M., Kovesi, T., Guerra, L. et al. The impact of pneumoperitoneum and Trendelenburg positioning on respiratory system mechanics during laparoscopic pelvic surgery in children: a prospective observational study. Can J Anesth/J Can Anesth 62, 798–806 (2015). https://doi.org/10.1007/s12630-015-0369-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-015-0369-0